platform basic account
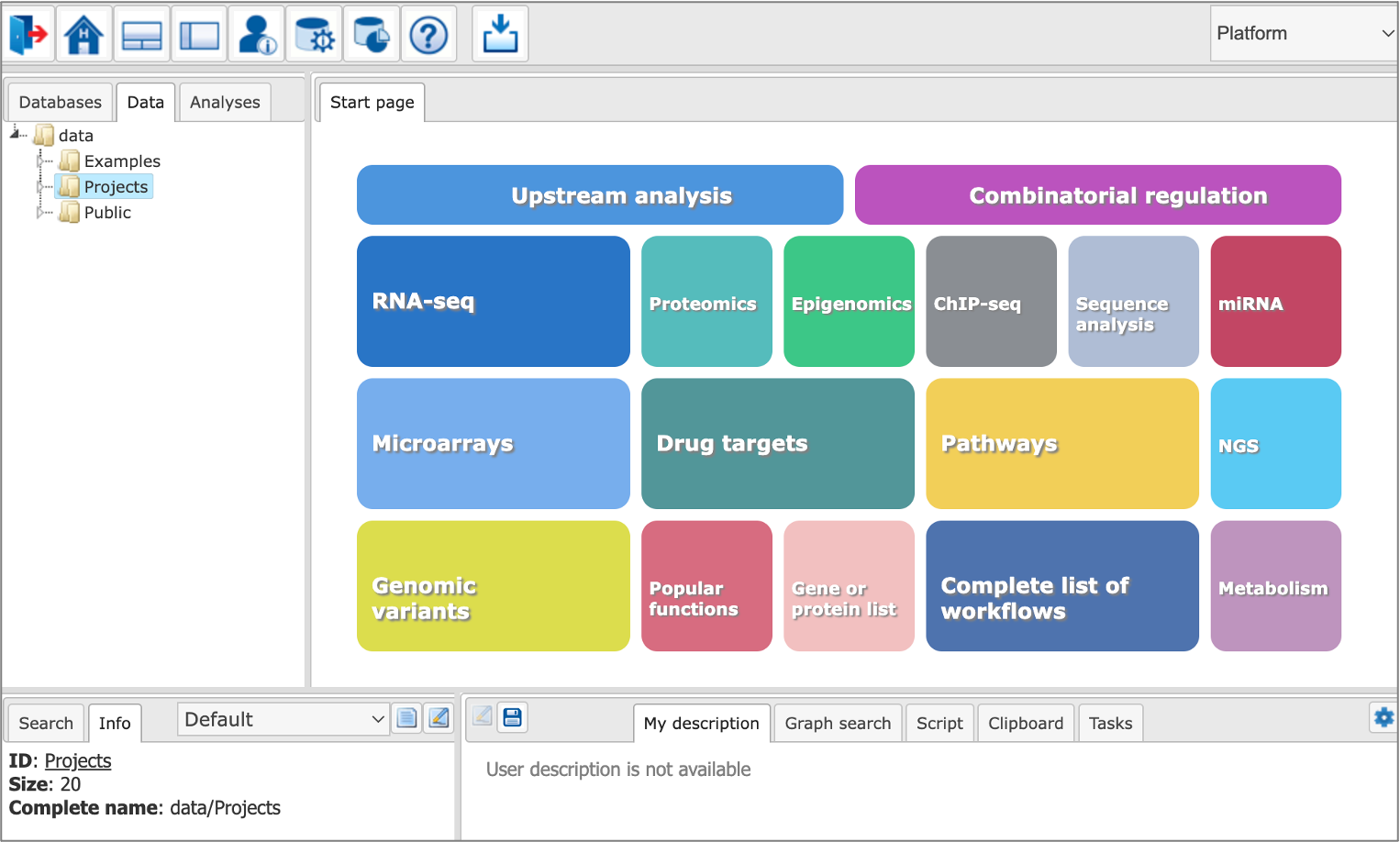
The geneXplain platform is a free online toolbox for your bioinformatic applications
Register via this form to immediately get your free geneXplain platform account.
Select the “Registration for a free platform account” option at the top of the form to proceed.
Please note that the free account provides 15 MB disk space for performing the analysis. In case you will exceed this limit, an email will be sent to the address you have provided upon registration and your account will be transferred to a read/delete access mode within 3 days. Starting from that point, you will have 30 calendar days to either delete your data to meet the free account limit of 15 MB, or purchase disk space from us. In case no further action will be taken by you within this period, all your data will be permanently deleted, after what your account will be reactivated.
If you will have any questions towards the geneXplain platform disk space purchasing procedure, please contact us via this form or by email inquiries@genexplain.com.