From Data to Discovery: The Story of LCTA – A Novel Class of Anticancer Compounds Targeting Thioredoxin Reductase
Cancer remains one of the greatest medical challenges of our time, and the development of new treatment strategies is crucial. At geneXplain, we have pioneered a unique computational methodology – upstream analysis – which has enabled us to identify and develop a new family of anticancer compounds, now patented under EP4201929. These compounds, collectively known as LCTA, represent a breakthrough because they exploit a rarely used mechanism of action: selective inhibition of thioredoxin reductase 1 (TrxR1), leading to fatal oxidative stress in tumor cells.
The Computational Beginning: Net2Drug and the Birth of Upstream Analysis
The origins of the LCTA project can be traced back to the Net2Drug project (2007–2010, FP6), coordinated by BIOBASE (now part of geneXplain). The goal of Net2Drug was to develop an integrated systems biology toolbox that could:
To achieve this, several computational and experimental layers were developed:
1. Promoter Analysis and TF Binding Sites
Using TRANSFAC® and novel algorithms such as P-Match and Composite Module Analyst (CMA), the consortium identified enriched transcription factor (TF) binding site motifs in the promoters of genes differentially expressed in breast cancer models. This enabled the detection of composite regulatory modules and cross-talk between TFs, a hallmark of cancer gene regulation.
2. Pathway Reconstruction and Master Regulator Identification
Leveraging the TRANSPATH® database and new graph-analysis methods, the project reconstructed disease-specific signaling pathways. Simulation of “dichotomic regulatory networks” allowed identification of key upstream regulators whose inhibition could collapse oncogenic signaling.
3. Integration of Multi-Omics and Experimental Data
4. Chemoinformatics and Virtual Screening
Using PASS and GUSAR, trained on ~30,000 compounds with antineoplastic activity, Net2Drug virtually screened 24 million molecules. This led to the selection of 64 high-confidence leads, of which 26 were experimentally available. Biological testing identified CPI, a compound with strong synergy with p53 activator RITA, as the first prototype for the family that would eventually evolve into LCTA compounds.
This data-to-drug pipeline—integrating promoter analysis, master regulator discovery, network modeling, and chemoinformatics—was the first demonstration of upstream analysis, later commercialized in our Genome Enhancer platform.
Refinement and Early Leads: The A-LAB Era
Building on Net2Drug, the A-LAB project (2012–2014) synthesized and tested derivatives of CPI, such as ALAB-1 and ALAB-2. These compounds were screened across diverse cancer cell lines and showed potent activity, including suppression of glioblastoma stem cells. A key discovery was LCTA2290, which significantly suppressed tumor growth in colon carcinoma xenografts and triggered transcriptional changes consistent with oxidative stress induction.
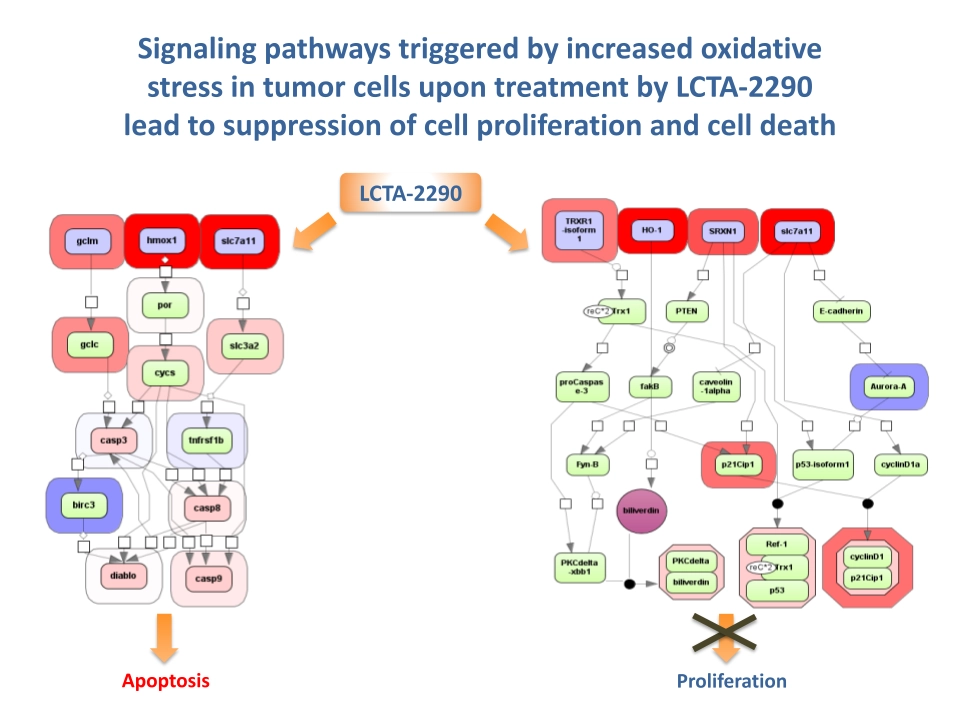
OxidoCurin: Optimization and Target Validation
The OxidoCurin Eurostars project (2018–2020) refined the compounds to address solubility and potency challenges.
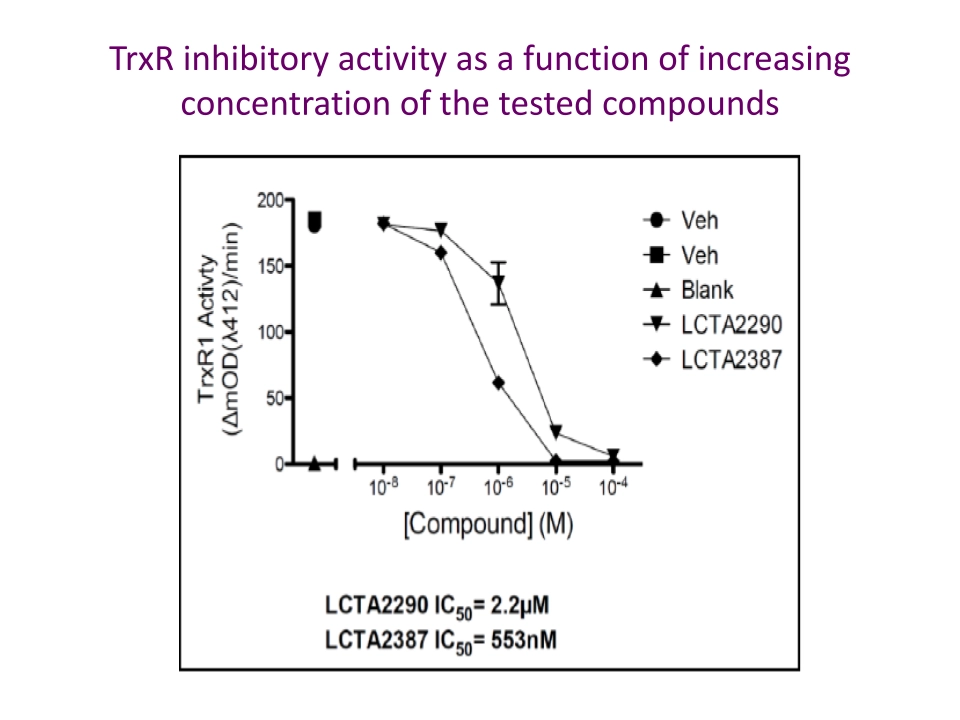
Experimental Proof: From Cells to Xenografts
The Latest Insights: Computational Analysis of LCTA2940
The 2022 final OxidoCurin report expanded our understanding of LCTA through advanced proteomics and computational biology:
Future Perspectives: Where LCTA Could Make the Biggest Impact
The LCTA family—through its targeted induction of oxidative stress via irreversible TrxR1 inhibition—opens up several promising therapeutic avenues, particularly in cancer types and contexts where ROS-based interventions are most effective.
Insights from Leading Experts in Redox-Targeted Drug Development
Prominent researchers in the field of cancer redox biology emphasize the dual nature of ROS in oncology: while modest ROS levels can foster tumor growth, surpassing a threshold can trigger apoptosis, especially when antioxidant defenses are compromised.
- Nakamura, H., et al.
Reactive oxygen species (ROS) as a “double-edged sword” in cancer: therapeutic strategies exploiting oxidative overload to push cancer cells beyond survival limits. ScienceDirect; PMC; MDPI. - Perillo, B., et al.
Modulating ROS levels to induce programmed cell death in tumor cells. Nature. - Seitz, R. (2024).
Targeting thioredoxin reductase (TrxR) as a selective therapeutic opportunity due to tumor dependence on antioxidant detoxification systems. IIAR Journals; PMC; Frontiers. - Bjørklund, G. (2021).
Thioredoxin reductase as a critical and clinically relevant pharmacological target in oncology. ScienceDirect. - Xu, Q. (2022).
Synergistic inhibition of the thioredoxin (Trx) system to overcome chemotherapy resistance and enhance immunotherapy responses. ScienceDirect; Taylor & Francis Online; Frontiers.
These expert perspectives strongly validate LCTA’s mechanism: selective TrxR1 inhibition is well-aligned with cutting-edge redox oncology strategies.
Strategic Future Indications for LCTA Compounds
1. Hypoxic Solid Tumors
Solid tumors such as pancreatic, lung, and glioblastoma often contain hypoxic regions with elevated ROS stress. LCTA2940’s heightened activity under hypoxia—demonstrated in xenograft models—makes it especially attractive for these challenging cancers.
2. Glioblastoma and Cancer Stem Cells
LCTA compounds have shown efficacy against glioblastoma stem cells—cells often spared by conventional treatments. Coupled with redox modulation strategies, they may help prevent recurrence and therapy resistance.
3. Hematological Malignancies Sensitive to Redox Disruption
LCTA exhibited potent activity in several leukemia and lymphoma lines, suggesting a role in blood cancers that rely on antioxidant systems for survival.
4. Combination Therapies to Overcome Resistance
For tumors with inherent or acquired resistance, LCTA could be combined with existing drugs to enhance oxidative burden. Aligning with Xu’s views on Trx system synergy, combinations with chemotherapy, radiotherapy, or immunotherapy could be particularly powerful.
5. Patient Stratification via Biomarkers
Proteomic analyses have identified markers such as PLK1, RELA (NF-κB p65), DMPK, mTOR, and HIPK2 as potential predictors of LCTA sensitivity. This enables precision oncology—identifying patients most likely to benefit from TrxR1-targeted therapy.
Summary Table of Potential Applications
|
Application Area |
Rationale & Supporting Evidence |
|
Hypoxic solid tumors |
Enhanced LCTA activity under hypoxia and vulnerability via ROS overload |
|
Glioblastoma/cancer stem cells |
Proven efficacy against therapy-resistant stem cells |
|
Hematologic cancers |
Broad activity across leukemia/lymphoma cell lines |
|
Combination therap |
Enhances efficacy and overcomes resistance through oxidative synergy |
|
Biomarker-guided treatment |
Proteomic signatures allow patient selection for better outcomes |
Final Thoughts
LCTA compounds—backed by solid mechanistic validation and promising preclinical efficacy—are ideally positioned to become the first-in-class TrxR1-targeted anticancer agents. By leveraging the inherent oxidative fragility of cancer cells and aligning with expert consensus on ROS-driven therapies, they hold the potential to transform treatment in:
Advancing LCTA into clinical development, accompanied by biomarker-guided patient selection, could offer a precision strategy that turns a cancer cell’s own oxidative weakness into a therapeutic advantage.
Conclusion
The story of LCTA demonstrates the full power of upstream analysis, a methodology born in Net2Drug and now fully automated in Genome Enhancer. From computational prediction to xenograft validation, the LCTA family highlights how systems biology + chemoinformatics + proteomics can accelerate drug discovery.
LCTA compounds exploit a rare vulnerability of cancer cells: their dependence on antioxidant defenses. By selectively shutting down TrxR1, LCTA induces lethal oxidative stress in tumors while sparing normal cells.
With patent protection, validated mechanism, in vivo efficacy, and biomarker insights, LCTA2940 is now poised for the next stage: clinical translation as a first-in-class TrxR1-targeted therapy.
