FOXO3: The Longevity Switch Inside Our Cells — Decoding the Master Regulator of Aging, Stress, and Disease
Introduction
Aging is a universal biological process, yet the reasons why some individuals live significantly longer and healthier lives have long puzzled scientists. Among the genes linked to exceptional longevity, FOXO3 consistently stands out as one of the most influential “master controllers” of cellular resilience. This single transcription factor integrates signals from stress, metabolism, DNA repair, and stem cell biology, orchestrating a vast genetic program that determines how cells survive, adapt, or age [1].
In recent years, interest in FOXO3 has surged across aging research, regenerative medicine, oncology, and precision therapeutics. Variants of the FOXO3 gene are strongly associated with centenarian populations worldwide, while disruptions in its regulatory network contribute to multiple disorders, including cancer, neurodegeneration, metabolic decline, and tissue degeneration. With advances in computational biology and pathway analysis, it is now possible to map FOXO3’s complex signaling network and uncover new therapeutic strategies.
This blog post explores FOXO3’s multifaceted biological roles, its influence on disease, and what our curated data from TRANSFAC®, TRANSPATH®, and HumanPSD™ reveals about the FOXO3 regulatory network. The goal is to provide a scientifically rich yet accessible overview that sparks curiosity among researchers studying aging, longevity, and systems-level biology.
Key functions and roles
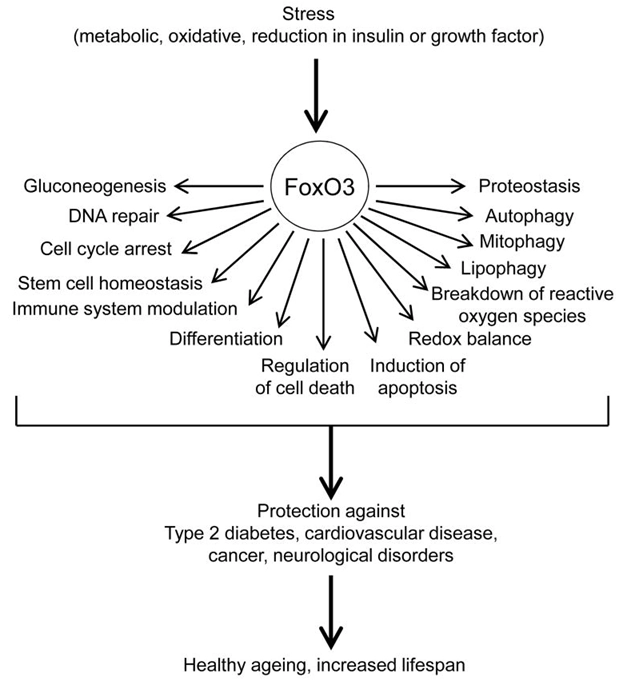
Longevity and Aging
Genetic variations in the FOXO3 gene are strongly associated with human longevity and healthy aging, making it a major gene linked to a longer lifespan [1]. FOXO3 SNPs are single-nucleotide polymorphisms in the FOXO3 gene that have been linked to human longevity and healthy aging. Several common variants, such as rs2802292, rs10457180, and rs2253310, are strongly associated with longevity across different populations [2]. FOXO3 is a key factor in aging, particularly in regulating vascular aging and protecting against age-related diseases [3]. FOXO3 slows down the aging process and exerts anti-aging effects by delaying telomere attrition, promoting cell self-renewal, and maintaining genomic stability [4].
Stress, Homeostasis & Cell Fate Decisions
FOXO3 helps the body adapt to stressors such as oxidative stress, DNA damage [5], and starvation. it does this by activating genes that promote cell survival, such as ROS scavengers and autophagy effectors, or genes that promote cell death and cell cycle arrest, depending on the stimulus and cellular context [2]. FOXO3 influences cell fate by acting as a transcription factor that regulates cellular processes like apoptosis, senescence, autophagy, and cell cycle arrest. The activity of FOXO3 is controlled by post-translational modifications and miRNAs, which can alter its location, stability, and DNA binding to determine the cell’s final outcome [6]. AMPK activation results in increased NAD + levels that lead to SIRT1 activation and deacetylation induced activation of its downstream targets like FOXO3 [7]. Activated FOXO3 inhibits the cell cycle by promoting the transcription of genes like p21 and p27, which inhibit cell division [8]. This is a key mechanism for its function as a tumor suppressor [9].
Autophagy Regulation
FOXO3 is a master regulator of autophagy, a process where a cell recycles its own components by degrading and recycling dysfunctional components. It controls autophagy primarily by moving into the cell’s nucleus during stress and activating a network of autophagy-related genes (e.g. Pink1, Bnip3 and Bnip3l) [10, 11].
Metabolic Control
FOXO3 regulates energy metabolism by influencing the expression of genes like TSC1, GS, and GLUD1 involved in glucose and glutamine utilization [12]. It is involved in regulating metabolism and protein turnover, and its activity can be influenced by the insulin/insulin-like growth factor signaling pathway, which can phosphorylate FoxO3, leading to its inactivation and exclusion from the nucleus. When insulin signaling is low (e.g., during starvation), FoxO3 can be activated to promote catabolic processes and stress resistance [13].
FOXO3 in Stem Cell Maintenance
FOXO3 is essential for maintaining and regenerating stem cells, which are vital for processes like wound healing and immune system function. FOXO3 is required for the expression of genes (p27 and Cyclin G2) involved in cell quiescence, it is necessary for the expression of genes involved in oxidative stress resistance (Selenbp1) and in glucose metabolism and transport (Pdk1, Slc2a3). The ability of FoxO3 to coordinate a program maintaining quiescence, stress resistance, and glucose metabolism is critical for preserving the stemness of the cells [14]. FOXO3 activates Notch Signaling and promotes the quiescent state during stem cell self-renewal in adult muscle regeneration [15].
In the sections below, we explore FOXO3’s multifaceted roles in cell fate decisions, stress resistance, metabolism, and disease, with focus on FOXO3 cellular signaling and on how decoding the FOXO3 network can inspire therapies for aging and cancer. Finally, we consider how using FOXO3’s network we can unlock new longevity-promoting and anti-cancer strategies. A dedicated analysis of “The FOXO3 Regulatory Network” highlights insights gained from GeneXplain’s proprietary tools and databases (TRANSFAC®, TRANSPATH®, etc.)
FOXO3 in Disease: Double-Edged Sword
FOXO3 acts as a tumor suppressor in most cancers by promoting cell death and inhibiting proliferation. FOXO3 is inactivated by mutation of the FOXO3 gene or cytoplasmic sequestration of FOXO3 protein. This inactivation is associated with the initiation and progression of cancer [16]. It is implicated in certain cancers, such as Acute myeloid leukaemia [17], Glioblastoma, Breast cancer, Prostate cancer [18], Glioma [19], Uveal melanoma [20], Colorectal cancer [21], Non-small cell lung cancer [22]. While generally a suppressor, FOXO3 has a role in promoting tumor growth in specific contexts, such as in Hepatocellular carcinoma [23], where high expression can be linked to a worse prognosis. FOXO3 plays a critical role in the metabolism of bone and cartilage. FOXO3 plays a protective role in intervertebral disc degeneration (IDD) by promoting the survival of disc cells and inhibiting matrix degradation, and its levels decrease with age and degeneration. When FOXO3 is activated, it can increase autophagy, reduce apoptosis, and help maintain the extracellular matrix, making it a potential therapeutic target for IDD. In contrast, low levels of FOXO3 lead to mitochondrial dysfunction, cell death, and a more severe degenerative process [24]. FOXO3 plays a complex and multifaceted role in neurodegenerative diseases, acting as both a protector and a promoter of neuronal death depending on the specific context. Dysregulation of FOXO3 is implicated in several neurodegenerative conditions, including Alzheimer’s. FOXO3 deficiency leads to increased cortical amyloid pathology, and Cdk5-mediated activation of FOXO3 is a significant contributor to the disease’s neurotoxicity in Alzheimer’s disease [25].
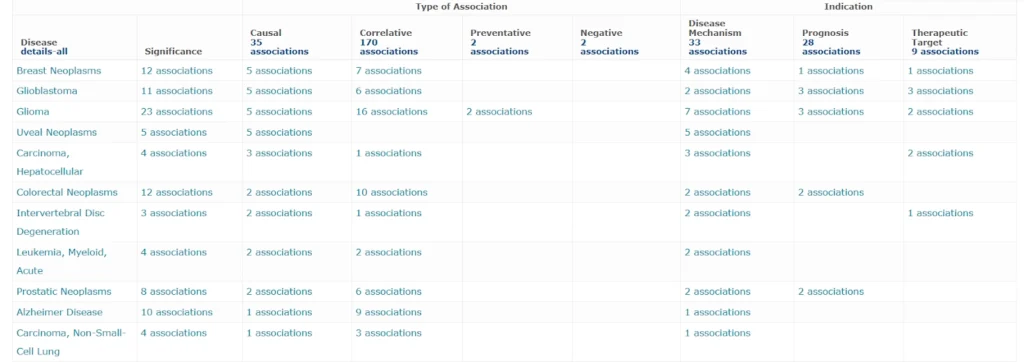
The table below presents associations of FOXO3 with several diseases. Each row of the table corresponds to a particular disease. In the columns the number of the associations is shown. This is a screenshot from the HumanPSD database, where all associations shown here are manually curated, and for each association more details are documented including the experimental details and the reference. In the HumanPSD release 2025.1, FOXO3 is documented to be associated with 70 different diseases, about half of them are different types of neoplasms.
The FOXO3 Regulatory Network: What Our Databases Reveal
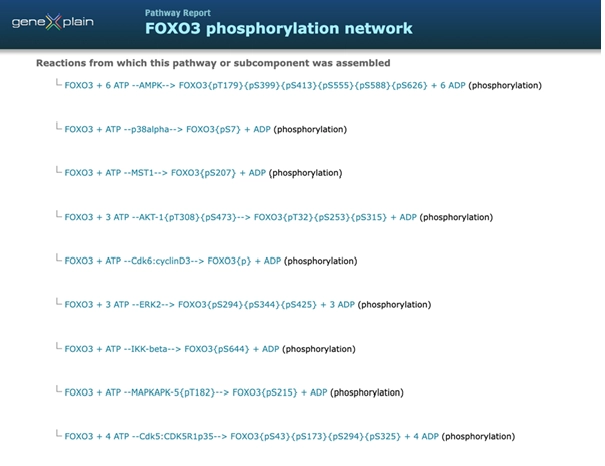
To understand FOXO3’s “master regulator” status, one must map the regulatory network around it. Using GeneXplain’s powerful databases and tools – including TRANSFAC® (transcription factor database) and TRANSPATH® (signal transduction pathway database) – we can find out how signals flow into and out of FOXO3. Several different kinases in TRANSPATH are noted to negatively regulate FOXO3. The activity of the FOXO3 transcription factor is controlled by a balance between its nuclear and cytoplasmic location, which is primarily managed through phosphorylation [26, 27, 28, 29]. All the facts mentioned below are manually curated in the TRANSPATH database, release 2025.1.
Under normal conditions, growth factors like insulin activate the PI3K/Akt signalling pathway. The activated Akt kinase phosphorylates FOXO3 on specific residues (at Thr32, Ser253, and Ser315) [27]. The phosphorylated FOXO3 protein binds to chaperone proteins called 14-3-3. This binding inhibits FOXO3 from entering into the nucleus, preventing it from activating its target genes. There are few more kinases which inhibit FOXO3 by phosphorylation, for example ERKs phosphorylate FOXO3( at Ser294, Ser344 and Ser425), SGK-1 phosphorylate FOXO3 (at Thr32, Ser253 and Ser315) [30]. IKK-beta also inhibits FOXO3 by promoting its phosphorylation [31].
When growth factors are low or absent, the PI3K/Akt pathway is inactive. This allows FOXO3 to remain unphosphorylated and translocate to nucleus.FOXO3 activity is also stimulated by stress-activated kinases, such as AMPK, which phosphorylates FOXO3 (at Thr179, Ser399, Ser413, Ser555, Ser588 and Ser626) and promote its nuclear localization [32]. In models of neurodegenerative diseases like Alzheimer’s, the aberrant activation of CDK5 by p25 phosphorylates FOXO3 (at Ser43, Ser173, Ser294 and Ser325) [33]. Phosphorylation of FOXO3 by MST1(at Ser207) and by p38 (at Ser7), inhibits the association of FOXO3 with 14-3-3 proteins, and promotes the nuclear translocation and transcription activity of FOXO3 [34, 35].
Once in the nucleus, FOXO3 regulates gene transcription. Using MATCH® with TRANSFAC, we can also examine transcriptional activity of FOXO3. FOXO3’s own promoter contains binding sites for several regulators, suggesting a network of control at the gene expression level. FOXO3 regulated genes control processes such as cell cycle arrest (Gadd45alpha, p27KIP1, cyclin D1 and cyclin D2), apoptosis (Bim, PUMA and Bcl-2, and stress resistance (Cat, MnSOD) [36,37].
On this screenshot from TRANSPATH database, release 2025.1, a summary of FOXO3 phosphorylation by different kinases is shown. All these phosphorylation reactions are manually curated in TRANSPATH including the experimental evidences and references.

Therapeutic implications:
The multifaceted nature of the FOXO3 pathway makes it an attractive target for therapeutic interventions, particularly in cancer and age-related diseases. Future therapies may focus on modulating FOXO3 through its upstream regulators, targeting its post-translational modifications, or using microRNAs to affect its expression [38]. However, its complex regulation means that activating or inhibiting it requires precise control to avoid side effects. GeneXplain’s Genome Enhancer pipeline can analyse patient omics data, identifying FOXO3 as a central hub disrupted in diseases, and giving an idea for a drug repurposing, which could be tested in further experiments. By analysing patient data and known drug profiles, these tools have highlighted candidates that influence FOXO3’s pathway. Genome Enhancer analysis can retrieve known small molecules that impact FOXO3 or its regulators. For example, Auranofin displays anticancer activity against ovarian cancer cells through FOXO3 activation.
FOXO3 is known to interact directly with several drugs, as shown on the screenshot from the HumanPSD database, release 2025.1

Conclusion
FOXO3 exemplifies how a single transcription factor can act as a central hub connecting stress response, metabolism, stem cell biology, aging, and disease. Its ability to integrate environmental and intracellular signals makes it one of the most powerful longevity-associated genes known to science. The curated knowledge compiled in TRANSFAC®, TRANSPATH®, and HumanPSD™ enables us to decode this network with unmatched depth — revealing how FOXO3 is regulated, how it influences cellular pathways, and how its dysfunction contributes to cancer, neurodegeneration, and age-related decline.
As research advances, FOXO3 is emerging not just as a marker of longevity but as a promising therapeutic target. Modulating its upstream regulators, controlling its post-translational modifications, and designing drugs that influence FOXO3’s signaling pathways may open new avenues for preventing age-associated diseases and extending healthspan. Understanding FOXO3 is, in many ways, a step toward understanding the molecular blueprint of a longer and healthier life.
References
- Morris BJ, Willcox DC, Donlon TA, Willcox BJ. FOXO3: A Major Gene for Human Longevity–A Mini-Review. Gerontology. 2015;61(6):515-25. doi: 10.1159/000375235. Epub 2015 Mar 28. PMID: 25832544; PMCID: PMC5403515.
- Sanese P, Forte G, Disciglio V, Grossi V, Simone C. FOXO3 on the Road to Longevity: Lessons From SNPs and Chromatin Hubs. Comput Struct Biotechnol J. 2019 Jun 13;17:737-745. doi: 10.1016/j.csbj.2019.06.011. PMID: 31303978; PMCID: PMC6606898.
- Chang ZS, He ZM, Xia JB. FoxO3 Regulates the Progress and Development of Aging and Aging-Related Diseases. Curr Mol Med. 2023;23(10):991-1006. doi: 10.2174/1566524023666221014140817. PMID: 36239722.
- Cao G, Lin M, Gu W, Su Z, Duan Y, Song W, Liu H, Zhang F. The rules and regulatory mechanisms of FOXO3 on inflammation, metabolism, cell death and aging in hosts. Life Sci. 2023 Sep 1;328:121877. doi: 10.1016/j.lfs.2023.121877. Epub 2023 Jun 22. PMID: 37352918.
- Bigarella CL, Li J, Rimmelé P, Liang R, Sobol RW, Ghaffari S. FOXO3 Transcription Factor Is Essential for Protecting Hematopoietic Stem and Progenitor Cells from Oxidative DNA Damage. J Biol Chem. 2017 Feb 17;292(7):3005-3015. doi: 10.1074/jbc.M116.769455. Epub 2016 Dec 19. PMID: 27994057; PMCID: PMC5314194.
- Khor YS, Wong PF. MicroRNAs-associated with FOXO3 in cellular senescence and other stress responses. Biogerontology. 2024 Feb;25(1):23-51. doi: 10.1007/s10522-023-10059-6. Epub 2023 Aug 30. PMID: 37646881.
- Chiacchiera F, Simone C. The AMPK-FoxO3A axis as a target for cancer treatment. Cell Cycle. 2010 Mar 15;9(6):1091-6. doi: 10.4161/cc.9.6.11035. Epub 2010 Mar 15. PMID: 20190568.
- Jie M, Wu Y, Gao M, Li X, Liu C, Ouyang Q, Tang Q, Shan C, Lv Y, Zhang K, Dai Q, Chen Y, Zeng S, Li C, Wang L, He F, Hu C, Yang S. CircMRPS35 suppresses gastric cancer progression via recruiting KAT7 to govern histone modification. Mol Cancer. 2020 Mar 12;19(1):56. doi: 10.1186/s12943-020-01160-2. PMID: 32164722; PMCID: PMC7066857.
- Stefanetti RJ, Voisin S, Russell A, Lamon S. Recent advances in understanding the role of FOXO3. F1000Res. 2018 Aug 31;7:F1000 Faculty Rev-1372. doi: 10.12688/f1000research.15258.1. PMID: 30228872; PMCID: PMC6124385.
- Deng A, Ma L, Zhou X, Wang X, Wang S, Chen X. FoxO3 transcription factor promotes autophagy after oxidative stress injury in HT22 cells. Can J Physiol Pharmacol. 2021 Jun;99(6):627-634. doi: 10.1139/cjpp-2020-0448. Epub 2020 Nov 25. PMID: 33237807.
- Audesse AJ, Dhakal S, Hassell LA, Gardell Z, Nemtsova Y, Webb AE. FOXO3 directly regulates an autophagy network to functionally regulate proteostasis in adult neural stem cells. PLoS Genet. 2019 Apr 11;15(4):e1008097. doi: 10.1371/journal.pgen.1008097. PMID: 30973875; PMCID: PMC6478346.
- Yeo H, Lyssiotis CA, Zhang Y, Ying H, Asara JM, Cantley LC, Paik JH. FoxO3 coordinates metabolic pathways to maintain redox balance in neural stem cells. EMBO J. 2013 Oct 2;32(19):2589-602. doi: 10.1038/emboj.2013.186. Epub 2013 Sep 6. PMID: 24013118; PMCID: PMC3791369.
- Jespersen JG, Nedergaard A, Reitelseder S, Mikkelsen UR, Dideriksen KJ, Agergaard J, Kreiner F, Pott FC, Schjerling P, Kjaer M. Activated protein synthesis and suppressed protein breakdown signaling in skeletal muscle of critically ill patients. PLoS One. 2011 Mar 31;6(3):e18090. doi: 10.1371/journal.pone.0018090. PMID: 21483870; PMCID: PMC3069050
- Renault VM, Rafalski VA, Morgan AA, Salih DA, Brett JO, Webb AE, Villeda SA, Thekkat PU, Guillerey C, Denko NC, Palmer TD, Butte AJ, Brunet A. FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell. 2009 Nov 6;5(5):527-39. doi: 10.1016/j.stem.2009.09.014. PMID: 19896443; PMCID: PMC2775802.
- Gopinath SD, Webb AE, Brunet A, Rando TA. FOXO3 promotes quiescence in adult muscle stem cells during the process of self-renewal. Stem Cell Reports. 2014 Mar 20;2(4):414-26. doi: 10.1016/j.stemcr.2014.02.002. PMID: 24749067; PMCID: PMC3986584.
- Liu Y, Ao X, Ding W, Ponnusamy M, Wu W, Hao X, Yu W, Wang Y, Li P, Wang J. Critical role of FOXO3a in carcinogenesis. Mol Cancer. 2018 Jul 25;17(1):104. doi: 10.1186/s12943-018-0856-3. PMID: 30045773; PMCID: PMC6060507.
- Hillion J, Le Coniat M, Jonveaux P, Berger R, Bernard OA. AF6q21, a novel partner of the MLL gene in t(6;11)(q21;q23), defines a forkhead transcriptional factor subfamily. Blood. 1997 Nov 1;90(9):3714-9. PMID: 9345057.
- Nho RS, Hergert P. FoxO3a and disease progression. World J Biol Chem. 2014 Aug 26;5(3):346-54. doi: 10.4331/wjbc.v5.i3.346. PMID: 25225602; PMCID: PMC4160528.
- Xu K, Pei H, Zhang Z, Dong S, Fu RJ, Wang WM, Wang H. FoxO3a mediates glioma cell invasion by regulating MMP9 expression. Oncol Rep. 2016 Nov;36(5):3044-3050. doi: 10.3892/or.2016.5087. Epub 2016 Sep 12. PMID: 27633065.
- Yan F, Liao R, Lin S, Deng X, Little PJ, Zheng W. Forkhead box protein O3 suppresses uveal melanoma development by increasing the expression of Bcl-2-like protein 11 and cyclin-dependent kinase inhibitor 1B. Mol Med Rep. 2018 Feb;17(2):3109-3114. doi: 10.3892/mmr.2017.8215. Epub 2017 Dec 7. PMID: 29257235.
- Bullock MD, Bruce A, Sreekumar R, Curtis N, Cheung T, Reading I, Primrose JN, Ottensmeier C, Packham GK, Thomas G, Mirnezami AH. FOXO3 expression during colorectal cancer progression: biomarker potential reflects a tumour suppressor role. Br J Cancer. 2013 Jul 23;109(2):387-94. doi: 10.1038/bjc.2013.355. Epub 2013 Jul 4. PMID: 23828518; PMCID: PMC3721407.
- Ebrahimnezhad M, Valizadeh A, Majidinia M, Tabnak P, Yousefi B. Unveiling the potential of FOXO3 in lung cancer: From molecular insights to therapeutic prospects. Biomed Pharmacother. 2024 Jul;176:116833. doi: 10.1016/j.biopha.2024.116833. Epub 2024 Jun 5. PMID: 38843589.
- Fondevila F, Fernández-Palanca P, Méndez-Blanco C, Payo-Serafín T, Lozano E, Marin JJG, González-Gallego J, Mauriz JL. Association of FOXO3 Expression with Tumor Pathogenesis, Prognosis and Clinicopathological Features in Hepatocellular Carcinoma: A Systematic Review with Meta-Analysis. Cancers (Basel). 2021 Oct 26;13(21):5349. doi: 10.3390/cancers13215349. Erratum in: Cancers (Basel). 2025 Jan 22;17(3):350. doi: 10.3390/cancers17030350. PMID: 34771514; PMCID: PMC8582569.
- Wang F, Wang Y, Zhang S, Pu M, Zhou P. YTHDF2-dependent m6A modification of FOXO3 mRNA mediates TIMP1 expression and contributes to intervertebral disc degeneration following ROS stimulation. Cell Mol Life Sci. 2024 Dec 3;81(1):477. doi: 10.1007/s00018-024-05503-w. PMID: 39625652; PMCID: PMC11615171.
- O’Mahony C, Hidalgo-Lanussa O, Barreto GE. Unveiling FOXO3’s metabolic contribution to menopause and Alzheimer’s disease. Exp Gerontol. 2025 Feb;200:112679. doi: 10.1016/j.exger.2025.112679. Epub 2025 Jan 9. PMID: 39778695.
- Huang H, Tindall DJ. Regulation of FOXO protein stability via ubiquitination and proteasome degradation. Biochim Biophys Acta. 2011 Nov;1813(11):1961-4. doi: 10.1016/j.bbamcr.2011.01.007. Epub 2011 Jan 14. PMID: 21238503; PMCID: PMC3110514.
- Dobson M, Ramakrishnan G, Ma S, Kaplun L, Balan V, Fridman R, Tzivion G. Bimodal regulation of FoxO3 by AKT and 14-3-3. Biochim Biophys Acta. 2011 Aug;1813(8):1453-64. doi: 10.1016/j.bbamcr.2011.05.001. Epub 2011 May 19. PMID: 21621563; PMCID: PMC3237389
- Yang W, Dolloff NG, El-Deiry WS. ERK and MDM2 prey on FOXO3a. Nat Cell Biol. 2008 Feb;10(2):125-6. doi: 10.1038/ncb0208-125. PMID: 18246039.
- Yang JY, Zong CS, Xia W, Yamaguchi H, Ding Q, Xie X, Lang JY, Lai CC, Chang CJ, Huang WC, Huang H, Kuo HP, Lee DF, Li LY, Lien HC, Cheng X, Chang KJ, Hsiao CD, Tsai FJ, Tsai CH, Sahin AA, Muller WJ, Mills GB, Yu D, Hortobagyi GN, Hung MC. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat Cell Biol. 2008 Feb;10(2):138-48. doi: 10.1038/ncb1676. Epub 2008 Jan 20. Erratum in: Nat Cell Biol. 2008 Mar;10(3):370. PMID: 18204439; PMCID: PMC2376808.
- Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol Cell Biol. 2001 Feb;21(3):952-65. doi: 10.1128/MCB.21.3.952-965.2001. PMID: 11154281; PMCID: PMC86685.
- Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, Kobayashi R, Hung MC. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004 Apr 16;117(2):225-37. doi: 10.1016/s0092-8674(04)00302-2. Erratum in: Cell. 2007 Jun 29;129(7):1427-8. PMID: 15084260.
- Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, Brunet A. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem. 2007 Oct 12;282(41):30107-19. doi: 10.1074/jbc.M705325200. Epub 2007 Aug 20. PMID: 17711846.
- Shi C, Viccaro K, Lee HG, Shah K. Cdk5-Foxo3 axis: initially neuroprotective, eventually neurodegenerative in Alzheimer’s disease models. J Cell Sci. 2016 May 1;129(9):1815-1830. doi: 10.1242/jcs.185009. Epub 2016 Mar 9. PMID: 28157684; PMCID: PMC4893801.
- Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villén J, Becker EB, DiBacco S, de la Iglesia N, Gygi S, Blackwell TK, Bonni A. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006 Jun 2;125(5):987-1001. doi: 10.1016/j.cell.2006.03.046. PMID: 16751106.
- Ho KK, McGuire VA, Koo CY, Muir KW, de Olano N, Maifoshie E, Kelly DJ, McGovern UB, Monteiro LJ, Gomes AR, Nebreda AR, Campbell DG, Arthur JS, Lam EW. Phosphorylation of FOXO3a on Ser-7 by p38 promotes its nuclear localization in response to doxorubicin. J Biol Chem. 2012 Jan 6;287(2):1545-55. doi: 10.1074/jbc.M111.284224. Epub 2011 Nov 29. PMID: 22128155; PMCID: PMC3256863.
- Fitzwalter BE, Thorburn A. FOXO3 links autophagy to apoptosis. Autophagy. 2018;14(8):1467-1468. doi: 10.1080/15548627.2018.1475819. Epub 2018 Jul 21. PMID: 29938591; PMCID: PMC6103664.
- Schmidt M, Fernandez de Mattos S, van der Horst A, Klompmaker R, Kops GJ, Lam EW, Burgering BM, Medema RH. Cell cycle inhibition by FoxO forkhead transcription factors involves downregulation of cyclin D. Mol Cell Biol. 2002 Nov;22(22):7842-52. doi: 10.1128/MCB.22.22.7842-7852.2002. PMID: 12391153; PMCID: PMC134724
- Orea-Soufi A, Paik J, Bragança J, Donlon TA, Willcox BJ, Link W. FOXO transcription factors as therapeutic targets in human diseases. Trends Pharmacol Sci. 2022 Dec;43(12):1070-1084. doi: 10.1016/j.tips.2022.09.010. Epub 2022 Oct 21. PMID: 36280450; PMCID: PMC12194985.
